Abstract
Introduction:
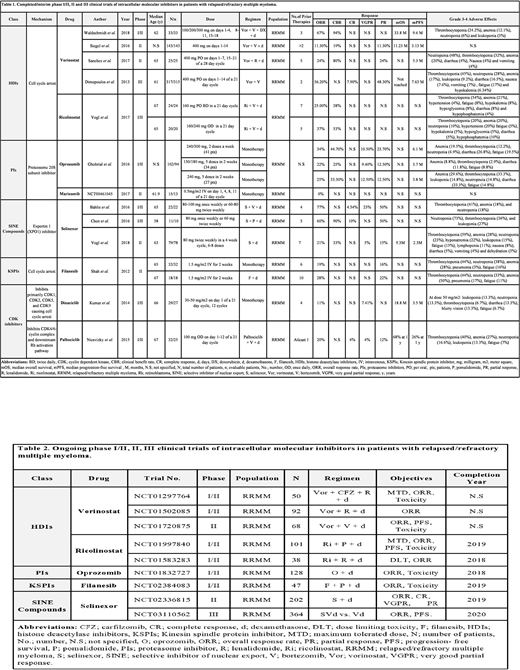
Intracellular molecular inhibitors act by inducing apoptosis due to inhibition of intracellular proteins like 20S proteasome subunit, histone deacetylases (HDAC's), exportin 1 (XPO1), cyclin-dependent kinases (CDKs) and kinesin spindle protein (KSP). These proteins are involved in the regulation of cell cycle. The aim of our analysis is to report published literature on the clinical efficacy of intracellular molecular inhibitors in relapsed and refractory multiple myeloma (RRMM) patients.
Methods:
Following Prisma guidelines, we performed a comprehensive literature search on articles published after 2011. Four hundred eighty-nine articles were identified using the following five databases (Pubmed, Embase Cochrane, Web of Science and Clinical Trials.gov). After a detailed screening, we finalized 13 studies involving 902 RRMM patients. We included phase I/II, II and III studies. The studies involving drugs approved by the US Food and Drug Administration were excluded.
Results:
Selective inhibitor of nuclear export (SINE) compounds: Selinexor
A total of 112 RRMM patients were included. Thirty-three patients were in phase I/II while 79 patients were in phase II. All patients received selinexor (60-100 mg) in different combination regimens. Forty-eight patients were quad-refractory (refractory to bortezomib, carfilzomib, lenalidomide, and pomalidomide) while 31 patients were penta-refractory (including isatuximab/daratumumab). In 110 evaluable patients, the pooled overall response rate (ORR) was 35.45%. The ORR in the subset of quad-refractory and penta-refractory patients was 21% and 20% respectively. The best response was seen when selinexor was used in combination with bortezomib (V) and dexamethasone (d) i.e. 77%.
HDAC inhibitors: Vorinostat and Ricolinostat
A total of 562 RRMM patients were included. Seventy-seven patients were in phase I/II, 168 patients in phase II, and 317 patients were in phase III. Five hundred and eighteen patients received vorinostat (100-400 mg) in different combination regimens while 44 patients received ricolinostat (160-240mg) in combination with Vd. The median follow-up period was 13.2-30.8 months. In 560 evaluable patients, the pooled ORR was 42.83% and 31.81% in patients who received vorinostat and ricolinostat, respectively. The best response was seen when vorinostat was used in combination with V, doxorubicin (DX) and d i.e. 67%.
Proteasome inhibitors: Oprozomib and Marizomib
A total of 117 RRMM patients were included. Hundred and two patients were in phase I/II while 15 patients were in phase II. Twenty-seven, 34 and 41 patients received single-agent oprozomib in the dose of 240 mg, 150-180 mg, and 240-300 mg respectively while 15 patients received single-agent marizomib (0.5 mg/m2). Out of 107 evaluable patients, the pooled ORR was 27.65% in patients who received oprozomib while no response was seen in patients who received marizomib however stable disease was seen in 31% of patients.
KSP inhibitor: Filanesib
A total of 50 RRMM patients were included. All patients were in phase II. Thirty-two patients received single-agent filanesib (1.5mg/m2) while 18 patients received filanesib (1.5mg/m2) in combination with d. The pooled ORR was 22%. The ORR was 19% when filanesib was used as a single agent while it was 28% when filanesib was used in combination with d.
CDK inhibitors: Dinaciclib and Palbociclib
A total of 61 RRMM patients were included. All patients were in phase I/II. Twenty-nine patients received single-agent dinaciclib (30-50 mg/m2) while 32 patients received palbociclib (100mg) in combination with Vd. In 52 evaluable patients, the ORR in patients who received dinaciclib was 11% while the ORR in patients who received palbociclib was 20%.
Conclusion:
In RRMM patients, vorinostat and selinexor when used in combination regimens demonstrated a weak efficacy with a pooled ORR of 43% and 36% respectively. The best response was seen in combination with Vd i.e. 67% and 77% respectively. The data on other intracellular molecular inhibitors (ricolinostat, oprozomib, marizomib, filanesib, dinaciclib, and palbociclib) when used either as single agents or in combination regimens, suggested a poor efficacy with an ORR of < 35%. However, future randomized well designed prospective clinical trials involving a larger population are required to further explore the efficacy of these agents in RRMM patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal